On the surface of it, Earth and Saturn's moon Titan are wildly different from one another. Earth is temperate and warmed by the Sun, liquid water flows across its surface, and life pervades its opulent biosphere. Titan is beyond the reach of the Sun's warmth, is frigid and lifeless, and orbits a gas giant that is also lifeless.
Yet they're both rocky worlds with thick atmospheres, and they're the only bodies in the Solar System where liquid flows across their surfaces. In Titan's case, the surface liquids are frigid hydrocarbons, not water, though it may have a subsurface water-ammonia ocean. Some researchers have wondered if some type of life could exist on Titan, where liquid hydrocarbons take over water's role in performing the functions of a cell.
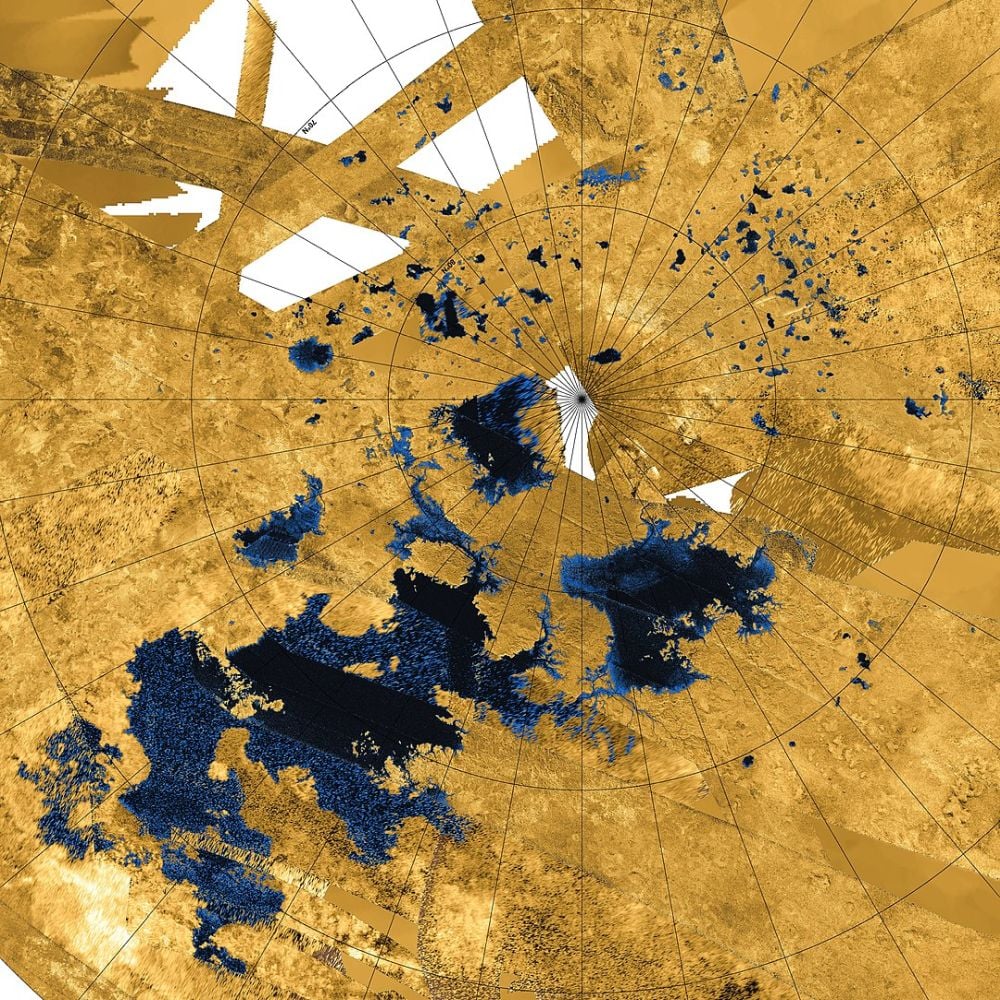 This Cassini radar image shows liquid hydrocarbon lakes, seas, and tributaries on Titan. It's the only other world besides Earth with liquid flowing on its surface. Image Credit: By NASA / JPL-Caltech / Agenzia Spaziale Italiana / USGS - http://photojournal.jpl.nasa.gov/catalog/PIA17655, Public Domain.
This Cassini radar image shows liquid hydrocarbon lakes, seas, and tributaries on Titan. It's the only other world besides Earth with liquid flowing on its surface. Image Credit: By NASA / JPL-Caltech / Agenzia Spaziale Italiana / USGS - http://photojournal.jpl.nasa.gov/catalog/PIA17655, Public Domain.
Scientists are interested in frigid Titan because of chemistry. The moon's chemistry, with its dense nitrogen and methane atmosphere, is similar to early Earth's atmosphere. This means it could serve as a sort of analog for early Earth, and studying it could reveal things about how Earth was billions of years ago, and by extension, clues to how life appeared.
New research in Proceedings of the National Academy of Sciences discovered a new way that certain substances can combine molecularly in Titan's extreme conditions. It's titled simply "Hydrogen cyanide and hydrocarbons mix on Titan." The lead author is Martin Rahm, Associate Professor at the Department of Chemistry and Chemical Engineering at Chalmers University of Technology in Sweden.
"This work uncovers the unexpected solid-state molecular mixing of nonpolar hydrocarbons—such as methane and ethane—with hydrogen cyanide (HCN), a compound more polar than water," the authors write in their research. This challenges chemical convention, according to the authors, and can shed new light on our understanding of chemistry in the Solar System. "It suggests different ways to think about chemical interactions with HCN at lower temperatures, potentially reshaping our understanding of processes in diverse environments across the solar system," they explain.
Polarity is a fundamental characteristic in chemistry. Polar compounds attract one another, and bonds between polar and nonpolar molecules are rare.
“These are very exciting findings that can help us understand something on a very large scale, a moon as big as the planet Mercury,” lead author Rahm said in a press release.
![This Cassini image of Titan has become iconic. The moon's thick organic atmosphere blocks visible light, limiting our attempts to understand its surface chemistry. Image Credit: By NASA/JPL-Caltech/SSI/Kevin M. Gill - File:Titan - December 16 2011 (40047599334).jpg by [1], CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=125680346](https://www.universetoday.com/article_images/Titan_in_true_color_by_Kevin_M._Gill_20251021_171531.jpg) *This Cassini image of Titan has become iconic. The moon's thick organic atmosphere blocks visible light, limiting our attempts to understand its surface chemistry. Image Credit: By NASA/JPL-Caltech/SSI/Kevin M. Gill - File:Titan - December 16 2011 (40047599334).jpg by [1], CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=125680346*
*This Cassini image of Titan has become iconic. The moon's thick organic atmosphere blocks visible light, limiting our attempts to understand its surface chemistry. Image Credit: By NASA/JPL-Caltech/SSI/Kevin M. Gill - File:Titan - December 16 2011 (40047599334).jpg by [1], CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=125680346*
Titan's chemistry is defined by vast quantities of the hydrocarbons ethane and methane, and the extremely toxic chemical hydrogen cyanide (HCN). The research shows that these chemicals can interact with one another in ways scientists didn't think was possible. HCN is highly polar, and the researchers found that it can form crystals with ethane and methane in Titan's frigid conditions.
"This work reveals a striking exception to the well-established rule in chemistry that polar and nonpolar compounds do not spontaneously mix: insertion of methane, ethane, and other small hydrocarbons into the crystal lattice of hydrogen cyanide (HCN), a highly polar molecule," the authors explain in their paper. This realization could extend to the appearance of life, since HCN plays a role in forming life's building blocks. It can form amino acids when interacting with ammonia and water.
“The discovery of the unexpected interaction between these substances could affect how we understand Titan’s geology and its strange landscapes of lakes, seas and sand dunes. In addition, hydrogen cyanide is likely to play an important role in the abiotic creation of several of life’s building blocks, for example amino acids, which are used for the construction of proteins, and nucleobases, which are needed for the genetic code. So our work also contributes insights into chemistry before the emergence of life, and how it might proceed in extreme, inhospitable environments,” said lead author Rahm.
The more we gaze into space and spy other solar systems and exoplanets, the more we are confronted with extreme environments. For most of these worlds, life is almost certainly impossible. But others are less extreme. Is it possible that there are other pathways to life on some of these distinctively non-Earthlike worlds? Maybe Titan's chemistry and conditions can help us understand.
An unanswered question played a role in this discovery.
Scientists know that HCN is created in Titan's atmosphere by photochemistry. Methane (CH4) and nitrogen (N2) are the two most abundant chemicals in Titan's atmosphere. When solar UV radiation and cosmic rays strike CH4 and N2, the molecules are broken apart. The fragments recombine into HCN and other products.
But what happens to all of the HCN? Does it gather on the planet's surface? A group of researchers at NASA's JPL ran laboratory experiments where they mixed HCN with methane and ethane at about -180 F. At that low temperature, HCN is a crystal and the hydrocarbons are liquids, just like on Titan's surface. When they examined their results, the molecules were intact, but something had changed. Since Rahm's research group at Chalmers University had researched HCN extensively, they turned to Rahm for assistance.
"This led to an exciting theoretical and experimental collaboration between Chalmers and NASA," said Rahm. "The question we asked ourselves was a bit crazy: Can the measurements be explained by a crystal structure in which methane or ethane is mixed with hydrogen cyanide? This contradicts a rule in chemistry, ‘like dissolves like’, which basically means that it should not be possible to combine these polar and nonpolar substances.”
Rahm and his colleagues turned to simulations to uncover some answers. They tested thousands of different ways that methane, ethane, and hydrogen cyanide could interact. They found that the pair of hydrocarbons had infiltrated the hydrogen cyanide crystal structure. This formed new, stable structures called cocrystals, which are an example of host-guest chemistry.
“This can happen at very low temperatures, like those on Titan. Our calculations predicted not only that the unexpected mixtures are stable under Titan’s conditions, but also spectra of light that coincide well with NASA’s measurements,” Rahm said.
The discovery doesn't negate our understanding of polar and nonpolar substance. Instead, it expands it. “I see it as a nice example of when boundaries are moved in chemistry and a universally accepted rule does not always apply,” Rahm said.
There are other recent examples of new chemical pathways being discovered on other worlds. For example, scientists detected phosphine in Venus' clouds. There are no known pathways for the abiotic creation of phosphine, so unless life is hiding somewhere on the uninhabitable planet, there must be an abiotic pathway that we're unaware of.
The JWST detected dimethyl sulfide (DMS) on exoplanet K2-18b, a compound and potential biosignature created by living things here on Earth. But in 2024 it was also found on the icy comet Comet 67P/Churyumov-Gerasimenko, showing that there must also be pathways to its creation that we simply don't know about yet. The takeaway is that our knowledge is incomplete.
Fortunately, our understanding of intriguing Titan is poised to take a grand leap. NASA's Dragonfly mission to Titan is scheduled to launch in 2028 and reach the frigid moon in 2034. Titan's thick atmosphere obscures much of the chemical signals from it surface, and so the details of its surface chemistry are largely unknown. Dragonfly will measure the compositions of materials on the moon's surface in detail, and will help us understand how far prebiotic chemistry can progress.
“Hydrogen cyanide is found in many places in the Universe, for example in large dust clouds, in planetary atmospheres and in comets. The findings of our study may help us understand what happens in other cold environments in space," Rahm said. "And we may be able to find out if other nonpolar molecules can also enter the hydrogen cyanide crystals and, if so, what this might mean for the chemistry preceding the emergence of life."
"Given that methane, ethane, and HCN are major components of the atmosphere and surface of Saturn’s moon Titan—where they play key roles in shaping chemistry, weather, and landscape—our findings may prove instrumental for explaining Titan’s chemical and geological evolution," the researchers conclude.

